Lecture 11
Dynamic Instability of the Microtubule
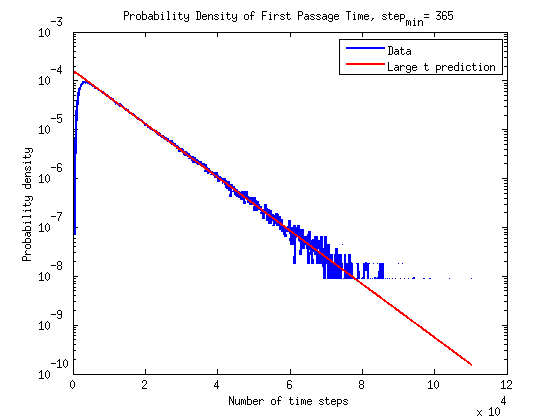
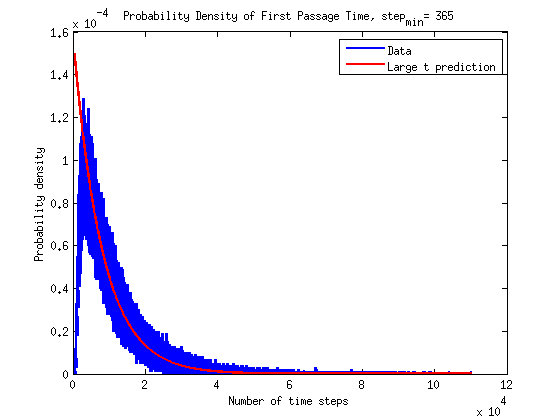
What is the timescale of fluctuations? ()
A good guess would be that it depends only on the two rates which we are aware of - the attach and detach rates.
Dynamic Instability in microtubule-like filaments
- Microtubules have dynamic instability
- One end of the microtubule is composed of stable (GTP) monomers while the rest of the tubule is made up of unstable (GDP) monomers.
- The GTP end comprises a cap of stable monomers.
- Random fluctuations either increase or decrease the size of the cap.
- This results in 2 different dynamic states for the microtubule.
- Growing: cap is present
- Shrinking: cap is gone
- This results in 2 different dynamic states for the microtubule.
- One end of the microtubule is composed of stable (GTP) monomers while the rest of the tubule is made up of unstable (GDP) monomers.
Lets define some parameters to describe how the microtubule switches between these to states and also how it behaves once in a state.
If we are in the growth state then monomers may be added to the filament.
If we are in the shrinkage state then monomers may be released from the filament.
The microtubule switches between these two states.
If we were to plot the length of the microtubule as a function of time it would look something like the following:
Lets now try to write a model that can describe how this occurs.
Dynamic Model
Lets define as being the fraction of time spent in the growth state. Hence the fraction of time spent in shrinkage state is then .
Similar to what we have done in previous lectures, our dynamic model is expressed as the following:
The fractions and can expressed in terms of the rates we defined above.
- Growth is bounded if .
- Growth is unbounded if .
Stochastic Model
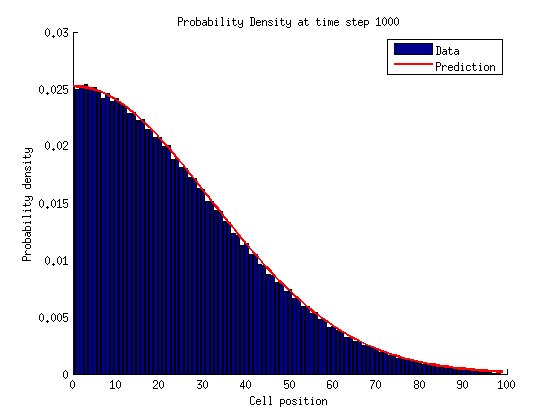
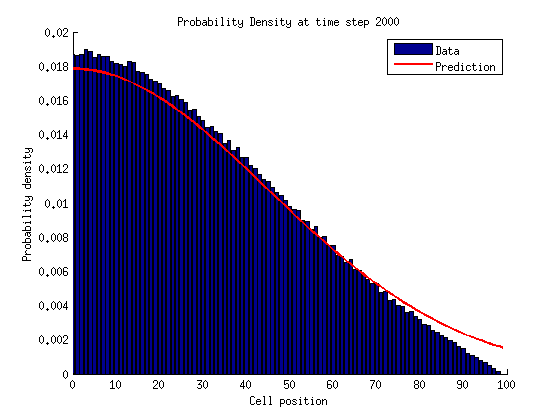
Now lets think in terms of probabilities. We need to assign probabilities to being in either a growth or shrinkage state with a certain number of links .
The probability of being a microtubule with links is .
If , .
If , .
Growth Rate
For
For
In a bounded state we can solve for the stationary distribution of by setting the time derivatives equal to zero.
For
For
If we repeat this process for we notice a pattern emerge.
We can solve for by normalizing the probability distribution.
This is not quite a (exponential) geometric distribution. A geometric distribution looks like the following:
Whereas in our case we have the following relationships:
Notice that when we solved for the series we assumed in order for the series to converge, and so this is in fact a bounded state.
Written with StackEdit.